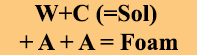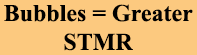All foams are wetting agents. Not all wetting agents are foam.
WATER (+’s and -‘s)
- + Absorbs Heat
- + Plentiful
- + Relatively Available
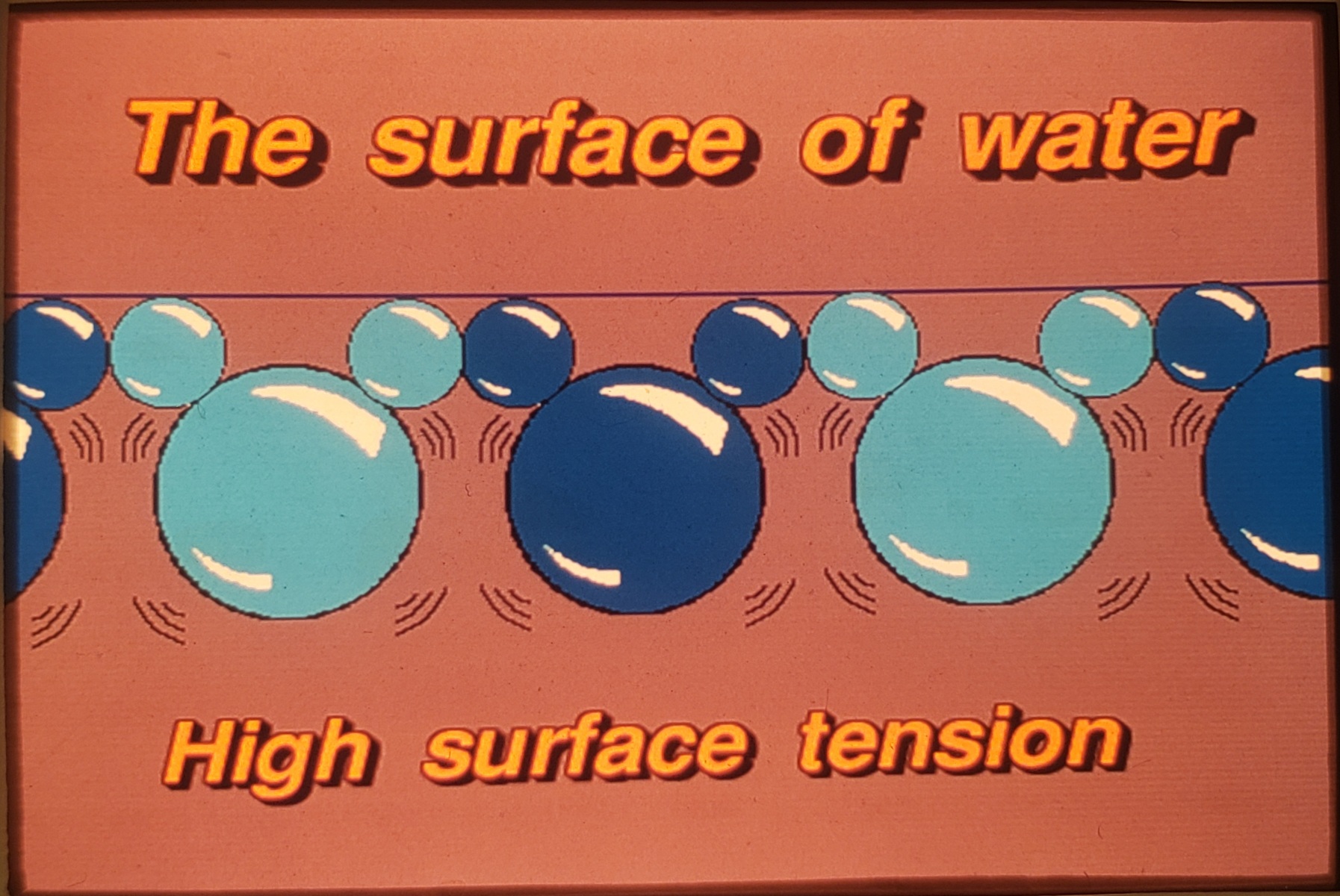
- – High Surface Tension (72 dynes/cm2) – Surface tension holds the water molecules together which limits water’s ability to absorb heat and limits the ability to penetrate or wet fuels.
- – Dislikes C (Carbon) – wil not bond with ordinary combustibles
- – Low Surface to Mass Ratio (STMR)
SURFACTANTS
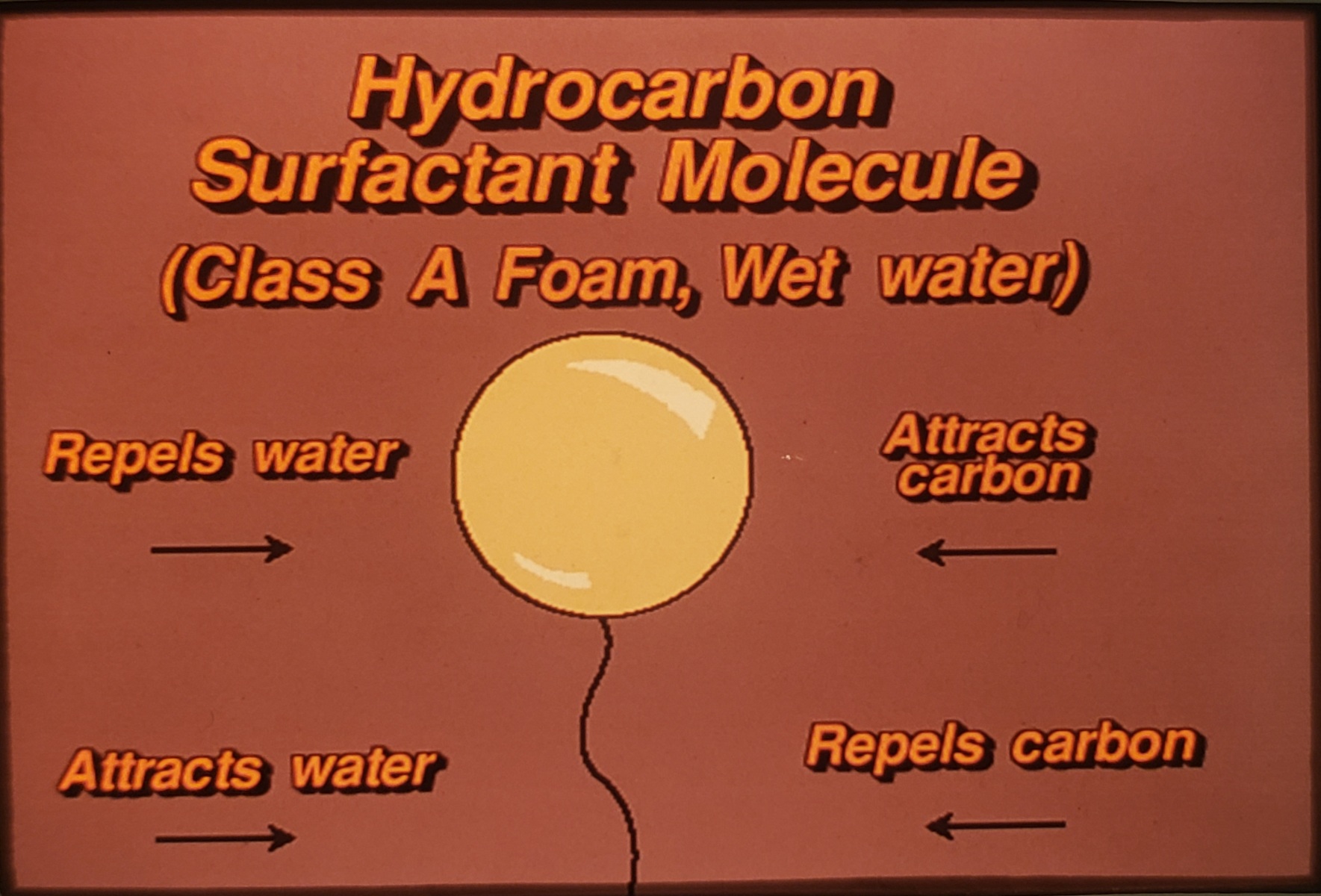
Synthetic, petroleum based, man-made, long-chain molecule product. (Added to water to make Foam Solution)
1/2 of the molecule – attaches to carbon and repels water
1/2 of the molecule repels carbon and attaches to water.
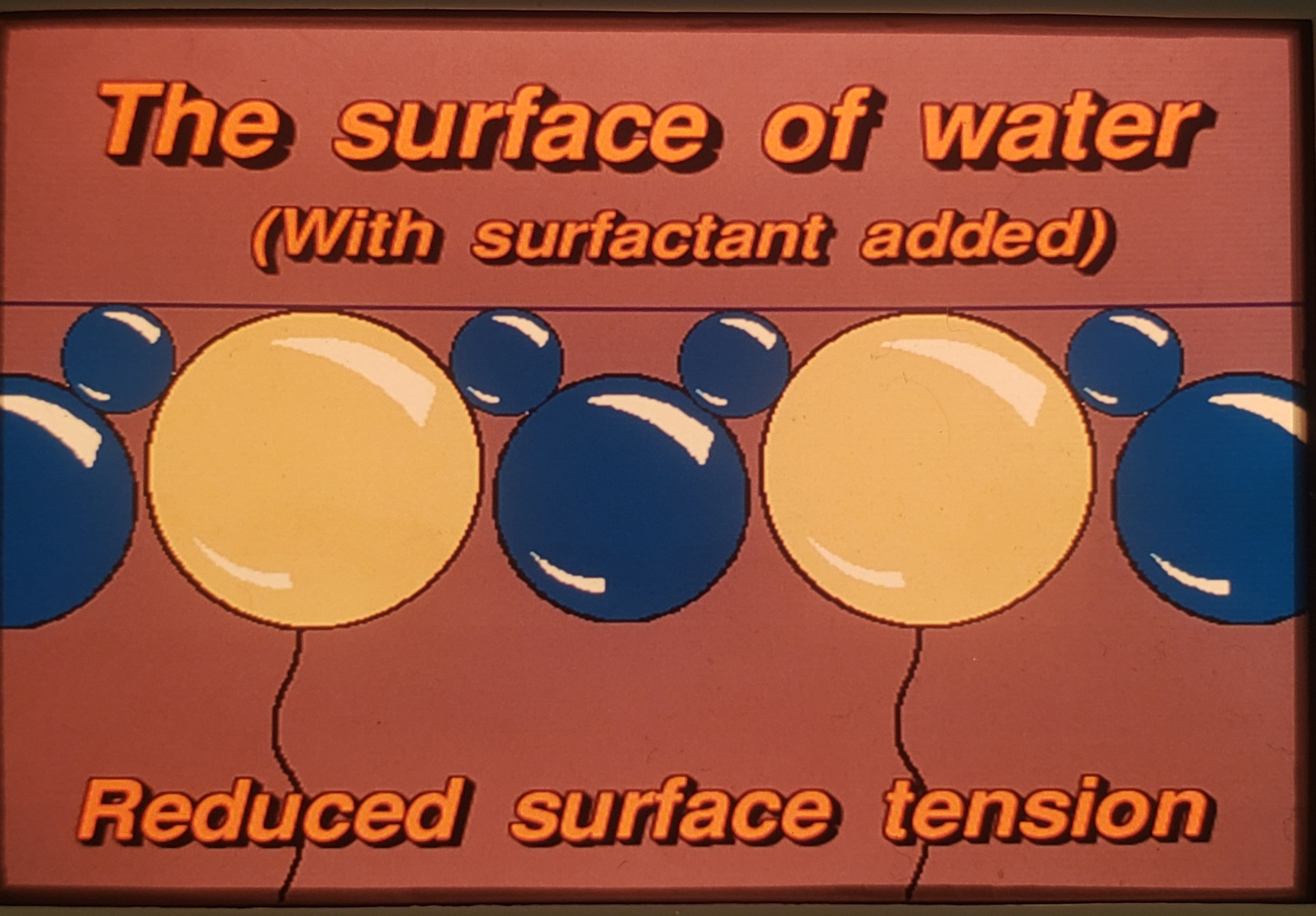
At the surface, the top of the molecule trys to repel the water while the lower half attaches to water. This causes the top of the water molecules to spread out, thus reducing surface tension. (25-30 dynes/cm2 – approximately 1/2 of untreated water). Water becomes wetter, spreads out faster and farther and soaks in (penetrates) 20x faster. Water can now cling to Carbon (Ordinary Combustibles).
Common examples of surfactants include Class A Foam, dish detergents, shampoos and some car was products to name a few.
There are two types of Class A surfactants:
- Wetting Agents
- Class A Foam Agents
Class A Foams are wetting agents, however, not all wetting agents are Foams. By definition, Foam is Bubbles. There are wetting agents that do not bubble, and while they will reduce surtface tension, they will not make FOAM.
Quality Class A Foams:
- Attach to Carbon
- Have reduced Surface Tension (wetting, speads and soaks in – penetrates)
- Create Bubbles (FOAM) – increased STMR {Link}
Make sure your department’s foam product meets all 3.